Dosing
- Two doses of SUFLAVE™ are required for a complete preparation
- Each dose consists of 1 bottle and 1 flavor enhancing packet
- Open 1 flavor-enhancing packet and pour the contents into 1 bottle.
- Fill the provided bottle with lukewarm water up to the fill line. After capping the bottle, gently shake the bottle until all the powder has dissolved. For the best taste, refrigerate the solution for 1 hour before drinking. Do not freeze. Use within 24 hours.
- Drink 8 ounces of solution every 15 minutes until the bottle is empty.
- Drink an additional 16 ounces of water during the evening.
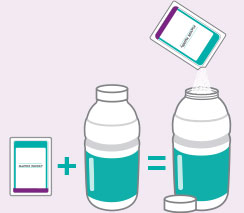 Bottles and packets not shown actual size.
Bottles and packets not shown actual size.
If nausea, bloating, or abdominal cramping occurs, pause or slow the rate of drinking the solution and additional water until symptoms diminish.
- Repeat Steps 1 through 3 from Day 1, Dose 1.
- Drink an additional 16 ounces of water during the morning.
- Stop drinking liquids at least 2 hours prior to colonoscopy.
 Bottles and packets not shown actual size.
Bottles and packets not shown actual size.
If nausea, bloating, or abdominal cramping occurs, pause or slow the rate of drinking the solution and additional water until symptoms diminish.

INDICATION
SUFLAVE™ (polyethylene glycol 3350, sodium sulfate, potassium chloride, magnesium sulfate, and sodium chloride for oral solution) is an osmotic laxative indicated for cleansing
of the colon in preparation for colonoscopy in adults.
DOSAGE AND ADMINISTRATION
A low residue breakfast may be consumed on the day before colonoscopy, followed by clear liquids up to 2 hours prior to colonoscopy. Administration of two doses of SUFLAVE™ are
required for a complete preparation for colonoscopy. Each bottle must be reconstituted with water before ingestion. Each bottle and one flavor enhancing packet are equivalent to
one dose. An additional 16 ounces of water must be consumed after each dose. Stop consumption of all fluids at least 2 hours before the colonoscopy.
CONTRAINDICATIONS
Use is contraindicated in the following conditions: gastrointestinal obstruction or ileus, bowel perforation, toxic colitis or toxic megacolon, gastric retention, hypersensitivity to any
ingredient in SUFLAVE™.
WARNINGS AND PRECAUTIONS
Risk of fluid and electrolyte abnormalities: Encourage adequate hydration, assess concurrent medications and consider laboratory assessments prior to and after each use;
Cardiac arrhythmias: Consider pre-dose and post-colonoscopy ECGs in patients at increased risk; Seizures: Use caution in patients with a history of seizures and patients at
increased risk of seizures, including medications that lower the seizure threshold; Colonic mucosal ulcerations: Consider potential for mucosal ulcerations when interpreting
colonoscopy findings in patients with known or suspected inflammatory bowel disease; Patients with renal impairment or taking concomitant medications that affect renal function:
Use caution, ensure adequate hydration and consider laboratory testing; Suspected GI obstruction or perforation: Rule out the diagnosis before administration; Patients at risk for aspiration: Observe during administration; Hypersensitivity reactions, including anaphylaxis: Inform patients to seek immediate medical care if symptoms occur.
ADVERSE REACTIONS
Most common adverse reactions (≥ 2%) are: nausea, abdominal distension, vomiting, abdominal pain, and headache.
DRUG INTERACTIONS
Drugs that increase risk of fluid and electrolyte imbalance.
View the Full Prescribing Information and Medication Guide.
References
1. SUFLAVE™ [package insert]. Braintree, MA: Braintree Laboratories, Inc.
2. Bhandari R, Goldstein M, Mishkin DS, et al. Comparison of a novel,
SUTAB® (sodium sulfate, magnesium sulfate, potassium chloride) tablets for oral use is an osmotic laxative indicated for cleansing of the colon in preparation for colonoscopy in adults.
CONTRAINDICATIONSUse is contraindicated in the following conditions: gastrointestinal obstruction or ileus, bowel perforation, toxic colitis or toxic megacolon, gastric retention, hypersensitivity to any ingredient in SUTAB®.
DOSAGE AND ADMINISTRATION
A low residue breakfast may be consumed. After breakfast, only clear liquids may be consumed until after the colonoscopy. Administration of two doses of SUTAB® (24 tablets) are required for a complete preparation for colonoscopy. Twelve (12) tablets are equivalent to one dose. Each SUTAB® bottle contains a desiccant. Remove and discard the desiccant from both bottles the evening prior to the colonoscopy. Water must be consumed with each dose of SUTAB® and additional water must be consumed after each dose. Complete all SUTAB® tablets and required water at least 2 hours before colonoscopy.
WARNINGS AND PRECAUTIONS
Risk of fluid and electrolyte abnormalities: Encourage adequate hydration, assess concurrent medications and consider laboratory assessments prior to and after each use; Cardiac arrhythmias: Consider pre-dose and post-colonoscopy ECGs in patients at increased risk; Seizures: Use caution in patients with a history of seizures and patients at increased risk of seizures, including medications that lower the seizure threshold; Patients with renal impairment or taking concomitant medications that affect renal function: Use caution, ensure adequate hydration and consider laboratory testing; Colonic mucosal ulcerations: Consider potential for mucosal ulcerations when interpreting colonoscopy findings in patients with known or suspected inflammatory bowel disease. Suspected GI obstruction or perforation: Rule out the diagnosis before administration. Hypersensitivity reactions, including anaphylaxis: Inform patients to seek immediate medical care if symptoms occur. Risk of Gastrointestinal Complications with Ingestion of Desiccant: Postmarketing reports of ingestion of the desiccant along with SUTAB® tablets has been reported and may be associated with risk of gastrointestinal complications and/or choking.
ADVERSE REACTIONS
Most common gastrointestinal adverse reactions are: nausea, abdominal distension, vomiting, and upper abdominal pain.
DRUG INTERACTIONS
Drugs that increase risk of fluid and electrolyte imbalance.
